Quantitative Viral Serology
5-What makes Allermetrix tests different from other laboratories?
The Allermetrix test is a laboratory developed test (LDT). Validation documents have been
submitted to the FDA and are available on the Allermetrix website.
Other current COVID-19 serology tests are qualitative which only tell you if you have
antibodies or not and many only test with one antigen. Allermetrix serology is quantitative
(reported in ug/mL and WHO international units) and indicates how much antibody you have
to three important COVID antigens;
o Receptor binding domain (RBD) which targets what cells get infected
o S1 subunit of the spike protein (S1) which aids in the infection process
o Nucleocapsid (N protein) which protects the viral RNA
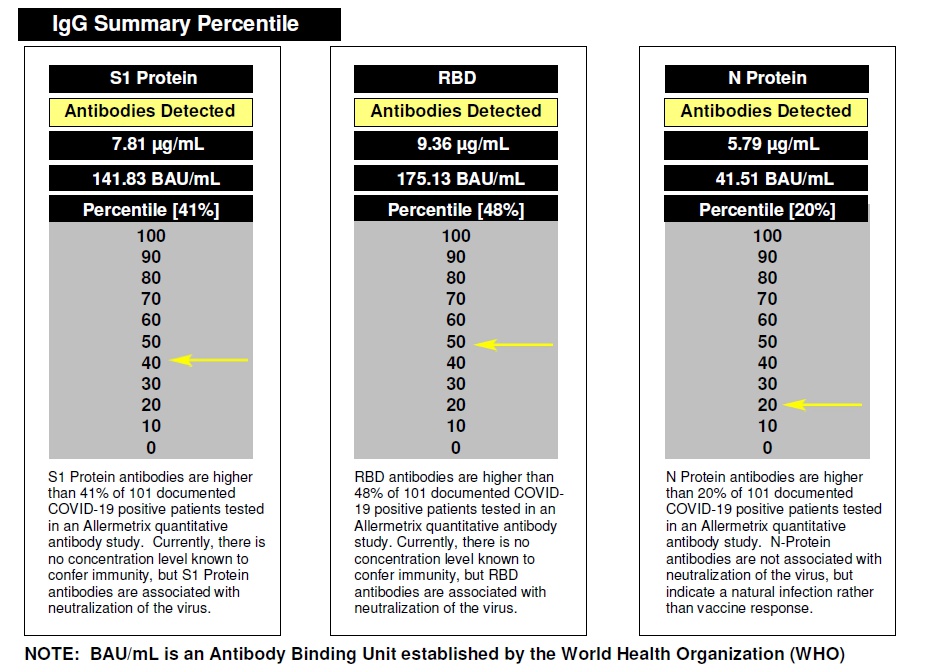
Allermetrix can show you how your results compare to samples with verified COVID-19
disease that we have tested. This is called benchmarking and an example of your report is
shown below: